-
Courses

Courses
Choosing a course is one of the most important decisions you'll ever make! View our courses and see what our students and lecturers have to say about the courses you are interested in at the links below.
-
University Life

University Life
Each year more than 4,000 choose University of Galway as their University of choice. Find out what life at University of Galway is all about here.
-
About University of Galway

About University of Galway
Since 1845, University of Galway has been sharing the highest quality teaching and research with Ireland and the world. Find out what makes our University so special – from our distinguished history to the latest news and campus developments.
-
Colleges & Schools

Colleges & Schools
University of Galway has earned international recognition as a research-led university with a commitment to top quality teaching across a range of key areas of expertise.
-
Research & Innovation

Research & Innovation
University of Galway’s vibrant research community take on some of the most pressing challenges of our times.
-
Business & Industry

Guiding Breakthrough Research at University of Galway
We explore and facilitate commercial opportunities for the research community at University of Galway, as well as facilitating industry partnership.
-
Alumni & Friends

Alumni & Friends
There are 128,000 University of Galway alumni worldwide. Stay connected to your alumni community! Join our social networks and update your details online.
-
Community Engagement

Community Engagement
At University of Galway, we believe that the best learning takes place when you apply what you learn in a real world context. That's why many of our courses include work placements or community projects.
Medical Technology Regulatory Affairs (MSc)
MSc (Medical Technology Regulatory Affairs)
College of Science and Engineering- Title of Award
- Master of Science
- Course Code
- MSC-MTR
- Delivery
- Online
- NFQ
- Level 9
- Award Type
- Major
- Duration
- 2 Years Part-time
- ECTS Weighting
- 90 ECTS
Why Choose This Course?
Course Information
The programme is offered as a two-year, part-time Level 9 MSc in Medical Technology Regulatory Affairs (90 credits). It is delivered online through distance-learning and e-learning technologies, with face-to-face workshops providing a blended learning experience. The programme consists of 12 modules, each worth 5 ECTS, with three modules delivered per semester. A 30-ECTS dissertation is completed across Years 1 and 2. Students are assessed through continuous assessment.
The MSc is also available in a modular format, allowing students to take individual modules or combinations of modules in any year, up to a maximum of three per semester. All 12 taught modules and the research project must be completed within six years to obtain the MSc degree. Postgraduate Certificate and Diploma exit awards are available upon successful completion of 6 or 12 taught modules.
Who is this course for?
This course is designed for professionals working in, or aspiring to enter, the Medical Technology, pharmaceutical, or related life-science sectors who wish to develop specialist expertise in Regulatory Affairs. It is ideal for those seeking career progression into regulatory, operations or quality roles, graduates aiming to build industry-relevant skills, and employees who need a recognised qualification to support their development in compliance, product approval, and regulatory compliance functions. It also suits individuals looking to transition into MedTech from scientific, engineering, or healthcare backgrounds and those seeking flexible, part-time study while working.
What will I study?
The programme is offered as a two-year, part-time Level 9 MSc in Medical Technology Regulatory Affairs (90 credits). It is also available in a flexible modular format, allowing students to take individual modules or combinations of modules in any year, up to a maximum of three per semester. Through this modular route, students may complete all 12 taught modules and the research project within six years to obtain the MSc degree. Postgraduate Certificate and Diploma exit awards are also available based on the completion of 6 or 12 taught modules.
The programme is delivered online using a blend of distance-learning and e-learning technologies, supported by occasional face-to-face workshops. Teaching is provided jointly by academic staff from Atlantic Technological University Sligo and the University of Galway, with additional contributions from industry specialists and practitioners.
The full MSc consists of 12 taught modules (5 ECTS each) delivered over two years—six modules per year—and a 30-ECTS research project completed across both years of study
Sample Delivery Schedule
Year 1 – Semester 1 (September–December)
Week 2 (September)
- 1-day on-campus student induction
Mid–Late November
- 1.5-day on-campus workshop covering all three Semester 1 modules (NOT compulsory)
Year 1 – Semester 2 (January–May)
Mid–Late March
- 1.5-day on-campus workshop covering all three Semester 2 modules (NOT compulsory)
Industry-driven curriculum, designed in collaboration with leading MedTech regulatory affairs experts and industry representatives.
Global regulatory focus, covering EU, US and key international markets.
Flexible, blended delivery – fully online distance learning with occasional face-to-face workshops; offered part-time over two years or in a modular format completed at your own pace (up to six years).
Practical, career-focused assessment, emphasising continuous assessment and real-world regulatory assignments.
Delivered jointly by University of Galway and ATU Sligo, supported by experienced practitioners and regulatory specialists.
A substantial 30-ECTS dissertation project, applying regulatory concepts to industry-relevant research challenges.
Supported by Medical Technologies industry representative organisations.
Careers
With a focus on real-world application, industry-aligned training, and a curriculum designed by regulatory experts, this programme prepares graduates for a wide range of career opportunities within the rapidly growing global Medical Technology sector.
Industry-relevant modules in areas such as Regulatory Submissions, Medical Device Quality Management, and International Regulatory Frameworks provide the specialised skills and professional competencies sought by employers navigating the increasingly complex MedTech regulatory landscape.
Graduates gain the expertise required to work across the entire medical-technology product lifecycle—supporting design, development, clinical trials, compliance, quality assurance, sterilisation, biocompatibility, market access, and post-market activities. The strong technical and regulatory foundation offered by this programme ensures career progression in roles such as Regulatory Affairs Specialist, Quality Assurance Associate, Compliance Officer, and Clinical Evaluation Specialist, and Post-Market Surveillance Analyst.
This programme also provides a strong base for further professional development and advanced study, supporting career advancement into senior regulatory, quality, or leadership roles.
Recent graduates and professionals with similar training have secured roles in leading MedTech companies across Ireland and internationally, including major employers in Galway’s world-renowned MedTech cluster.
Dr Olivia McDermott (University of Galway)— Associate Professor/Course Co-Director (University of Galway)
- Ms Mary Butler (ATU Sligo)—Co-Director
- Ms Deidre Barrow (Lecturer ATU Sligo)
- Dr Aidan Toner (Lecturer -University of Galway)
- Niamh Nolan (Lecturer -University of Galway)
- Dr Ailish Breen (ATU Sligo)—Lecturer
- Dr Mary Garvey (ATU Sligo)—Lecturer
- Dr Tom Patton (ATU Sligo)
- Dr Colin Fowley (ATU Sligo)
The MSc in Medical Technology Regulatory Affairs combines innovative teaching methods with practical, hands-on learning to ensure a comprehensive educational experience. The programme is delivered online through a combination of distance-learning and e-learning technologies, complemented by some face-to-face workshops (non compulsory) to provide an overall blended learning approach. The educational content will be delivered by staff from Atlantic Technological University, Sligo and the University of Galway. Additional lecturing, where required, may be provided by external specialists and industry practitioners.
How Will I Be Assessed?
Assessment will be carried out through continuous assessment across each module. This will include a combination of written assignments, team-based assessments, and open-book examinations incorporating multiple-choice questions, matching questions, and both short and long answer formats. In addition, students will be assessed on a Research Project thesis, which evaluates their ability to apply knowledge and skills to an in-depth, industry-relevant topic.
The University of Galway Course Director Dr Olivia McDermott is on the Elsevier/Stanford University “Top 2%” Top Scientists list for her research.
Over 20% of our graduates from the course have published their dissertation work in top ranking Q1 and Q2 peer reviewed journals in the Operations, Quality Management and Regulatory Field.
-Several former course graduates are completing part time doctoral research (PhD) in Dr McDermotts research group
Course Director (Galway):
Dr Olivia McDermott
E: olivia.mcdermott@universityofgalway.ie
Course Director (Sligo):
Ms Mary Butler
Atlantic Technological University, Sligo
E: mary.butler@atu.ie
Administrator (Galway):
medtech@universityofgalway.ie
The University of Galway recognises that knowledge and skills can be acquired through a wide range of learning experiences. This aligns with the goals of the National Framework of Qualifications (NFQ), which seek to recognise all learning achievements by supporting the development of alternative pathways to qualifications and facilitating the Recognition of Prior Learning (RPL).
Prior experience within the medical technology industry, particularly in regulatory affairs, will be considered a distinct advantage when assessing applications. Applications will also be considered from candidates who hold a Second Class Honours Level 8 primary degree in a non-technical discipline and who have a minimum of five years’ experience in the medical technology industry in quality and/or regulatory affairs.
Graduates of this programme will demonstrate the ability to:
- Apply comprehensive regulatory knowledge and professional competence across complex, highly regulated environments
- Conduct advanced data analysis, critical evaluation, and accurate interpretation of scientific information
- Communicate complex scientific and technical data clearly and persuasively to diverse stakeholders, including regulators, healthcare professionals, internal teams, and the public
- Adapt effectively to changing regulatory frameworks and evolving medical technologies through strong problem-solving and decision-making skills
- Provide strategic, tactical, and operational support within multidisciplinary teams to ensure the timely development and delivery of compliant, safe, and effective products
- Exercise professional judgement in risk management, compliance planning, and regulatory strategy implementation
- Demonstrate leadership, organisational and project management skills in regulated industry settings
- Translate scientific evidence into practical, regulatory-compliant recommendations and solutions
Accreditations & Awards
Meet our Employers
Entry Requirements and Fees
- Candidates should hold at least a Second Class Honours Level 8 primary degree in a relevant subject area in science or engineering
- Prior medical technology industry experience in regulatory affairs will be considered a distinct advantage in assessing applications
- Applications from candidates who hold a Second Class Honours Level 8 primary degree in a non-technical subject, and who have at least five years medical technology industry experience in quality and/or regulatory affairs will be considered
- Applications will also be considered from candidates who hold a degree in a relevant subject area in science or engineering at Level 7, with at least two years’ medical technology industry experience in quality and/or regulatory affairs
- Candidate interviews may be used to assess candidates’ suitability for the programme
- International students, whose first language is not English, will be required to prove their English competency through their school leaving examination or matriculation examination or by achieving the minimum standard in a recognised English language test
English Language Entry Requirements (replace/edit the text below)
For applicants whose first language is not English, an English language proficiency of IELTS score of 6.5 is required (with no less than 6.5 in Writing and no less than 6.0 in any other band) or equivalent.
More information on English language test equivalency are available here.
Supporting Documents (replace/edit the text below)
For all Science and Engineering Taught programmes the following
supporting documentation is required:
- Official qualifications and third level exam results (transcripts) to date: required for all non-University of Galway applicants and for University of Galway graduates who did not receive their undergraduate degrees from University of Galway. Applicants who have still to graduate must upload an interim transcript (of results to date) and upload final transcripts on receipt.
- English language competency: if necessary, evidence of English language competency.
- Personal statement: all applicants must enclose/upload a typed personal statementof approximately 600 words explaining why you wish to undertake the programme(s) of your choice, outlining how it fits into your career objectives, unless indicated otherwise in the table below.
- Passport or birth certificate:—a copy of your passport or birth certificate must also be submitted/uploaded.
- References: School of Engineering/School of Computer Science programmes—unless specifically stated otherwise non-University of Galway applicants only must provide two written references. One must be an academic reference (in the case of applicants currently undertaking studies) OR an employer (in the case of applicants currently in employment).
- IMPORTANT: Some programmes require additionalPlease view the list below for the list of programmes that require additional supporting documents.
Any application queries should be emailed to medtech@universityofgalway.ie
| Course Type | Year | EU Tuition | Student Contribution | Non-EU Tuition | Levy | Total Fee | Total EU Fee | Total Non-EU Fee |
|---|
Any application queries should be emailed to medtech@universityofgalway.ie
Why University of Galway?
World renowned research led university nestled in the vibrant heart of Galway city on Ireland's scenic West Coast.
Downloads
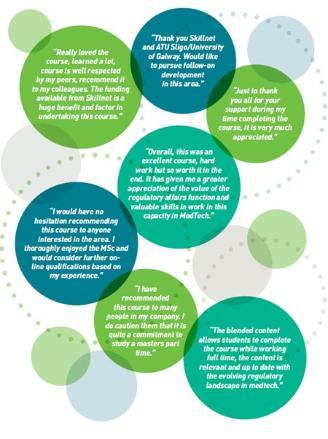
Meet Our Alumni
Course Introduction
This programme equips graduates with key skills for Regulatory Affairs roles in the global MedTech sector. Developed with industry experts, it addresses growing demand for regulatory and quality professionals. Regulatory Affairs is a dynamic, international field, essential across the product lifecycle in Ireland’s expanding MedTech industry.
Brochure
















.jpg)








